What is CGM? Simply standing for continuous glucose monitoring, CGM has taken a long time to be truly appreciated. Considered expensive, only with increased usage by both HCPs becoming familiar with the technology as well as diabetics wearing it, the full benefits are now coming to light. They are, at present, ‘as well as’, not ‘instead of’ normal blood tests. This is an overview of what is available in the UK. By Sue Marshall
It’s being proven through greater uptake and usage of devices that CGM is a support to the normal talks of blood testing and insulin delivery. Often associated with wearing an insulin pump, which act as receivers for the CGM data that is generated, it is often thought that if you use a CGM you no longer blood test, but that’s simply not the case. CGM sensors need regular calibrations from blood test machines. Worn on the body, CGM can be prone to snagging or can be a challenge to keep in place for the required period of time (usually 6 or 7 days) but over time that too becomes less of a worry as users get used to having CGM onboard.
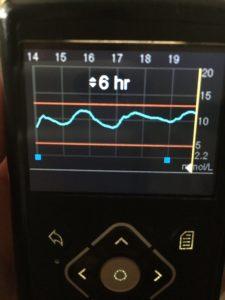
CGMs have a set of alarms that can be set up, alerting the wearer to trends of blood glucose going up, or down. The idea being that if you can detect and prevent these we can go a long way toward reducing the long-term complications that come from high and low blood sugars.
Most existing continuous glucose sensors work via an injection of an enzyme called glucose oxidase, which breaks down glucose. An electrode placed on the skin interacts with a by-product of that reaction, hydrogen peroxide, allowing glucose levels to be indirectly measured.
All aboard
CGM should not be confused with Flash Glucose Monitoring which is the technology behind Abbott’s FreeStyle Libre sensor (more of that below).
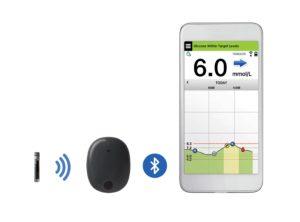
So how much CGM is out there? There are not many players in this exclusive field. Medtronic has been at the forefront of developing sensor technology that works with its own range of insulin pumps. As part of the move of CGM sensors away from solely serving people on pumps, Medtronic is opening it’s Enlite sensor and reader to a wider audience in a package called Medtronic Guardian Connect that does not work with an insulin pump but with a smartphone.
This consists of an Enlite sensor worn on the body that uses a Guardian Link to transmit glucose results every five minutes via Bluetooth to a free app on a smartphone, currently only iOS (Apple technology) with Android coming soon.
Back to the sensor-and-pump combination, Medtronic also has Smart Guard technology which operates on it’s MiniMed 640G insulin pumps. This incorporates ‘suspend before low’, whereby the pump is enabled to suspend insulin delivery when sensor readings go below an agreed level. This has been proven to greatly reduce – and even eliminate – hypos, especially at night giving users greater confidence in trying to keep glucose readings in range and thereby leading to greater overall control.
The purpose of the new Medtronic Guardian Connect is for people who either don’t want to pump, can’t get a pump, or have Type 2 diabetes and are not being encouraged to use an insulin pump.
Both options include a system of alarms that can be adjusted around an individuals needs and which have been proven to increase time spent in range. The alarms can help users eliminate being too high or too low. Using a smartphone means much more visibility of the data for the user (or interested parties — the system can also be made to send SMS alerts to parents, partners or HCPs.). However, you can’t control the pump from the app (if that is what you are using), nor is low glucose suspend available as that only works with a 640G insulin pump. The system needs calibrating at least twice a day; you just plug the calibration from your blood test meter into the app.
Medtronic’s Guardian Connect can be used alongside other pumps – as a stand-along system, it does not have to only work with Medtronic’s own insulin pumps. You can set up your own alerts and you’ll see arrows showing you if your readings are trending up or down.
No cause for alarm
Meanwhile, Dexcom has been making a lot of waves in terms of pushing awareness and availability of CGM forward. While not having an associated insulin pump of it’s own, the push has been to work towards integration with other pump suppliers. Notably, it’s G5 model has been cleared by the FDA in America for insulin dosing to be allowed based on a CGM reading. The FDA stated in December 2016 that, “The G5 Mobile Continuous Glucose Monitoring System uses a small sensor wire inserted just below the skin that continuously measures and monitors glucose levels. Real-time results are sent wirelessly every five minutes to a dedicated receiver and a compatible mobile device (e.g., smart phone or tablet) running a mobile app. Alarms and alerts indicate glucose levels above or below user-set thresholds. The system measures glucose in fluid under the skin and must be calibrated at least two times per day using blood obtained from fingerstick tests. However, additional daily fingerstick blood tests are generally no longer necessary because unlike other continuous glucose monitoring systems, results from this device can now be used directly by patients to make diabetes treatment decisions without confirmation from a traditional fingerstick test.”
Proof of positive impact of CGM for diabetics on injections has been shown via the DIaMonD study (Multiple Daily Injections and Continuous Glucose Monitoring in Diabetes), the first-of-its-kind study that demonstrated the impact of CGM on HbA1c and hypoglycemia in participants on a multiple daily injection (MDI) insulin regimen.
Published in January 2017 in Journal of the American Medical Association (JAMA), the study showed Dexcom CGM System users on MDI achieved a 1% average reduction in HbA1c after 24 weeks of regular use. In addition to better glucose control, participants also increased time spent in target range and spent less time in hypo and hyperglycemia when they used a Dexcom CGM system, compared to those who used only a standard meter to monitor their glucose. There was a 49% reduction in time spent in hypo (less than 3.9 mmol/L), a 53% reduction in time spent at less than 3.3 mmol/L and a 69% reduction in time spent at less than 2.8 mmol/L.
The study also dispelled certain perceptions, such as CGM being too complicated to use, as patients demonstrated significant HbA1c reductions regardless of education level, maths ability, or age. A high level of adherence was also achieved with 93% of patients still using the Dexcom CGM system more than at the end of the study.
Back in the US, Dexcom has recently announced that it will be working with health-wear providers Fitbit so that its CGM users will be able to see CGM data on Fitbit’s newest item, the Ionic Smartwatch, providing a convenient and discreet way to track glucose levels on wrist for users of both Android and iOS devices to help people better manage their diabetes and get a more complete picture of their overall health with easy-to-use mobile tools. “The collaboration between Dexcom and Fitbit is an important step in providing useful information to people with diabetes that is both convenient and discreet,” said Kevin Sayer, President and CEO, Dexcom. In addition to the Dexcom CGM display for Fitbit Ionic, with Fitbit’s in-app Community, Dexcom CGM users ought to be able to connect with millions of people with whom they can ask questions, seek support and share successes in managing their health. The companies are targeting availability as soon as possible in 2018.
Along came Abbott
Then a few years ago along Abbott and lobbed a bit of a grenade into the diabetes care arena with its FreeStyle Libre technology using Flash Glucose Monitoring*. CGM was not that well known or understood at the time, both in the diabetic user base and in diabetes healthcare. Perhaps without realizing it, Abbott made the use of sensors a much wider topic of debate, expanding the understanding of what can be done with more data.
And the timing of the launch of the new piece of kit coincided with social media being the ideal arena for people to share pictures of their reader’s screens – pronouncing it a good day, bad day and proving once and for all that having a ‘flat line’ is cool, so long as it’s in a healthy blood glucose range, and also that having a line that looks like a particularly uneven part of a mountain range is also normal for many a diabetic. It’s helped to show
The latest news about it being available on the NHS means that it can be accessed by a much wider range of people with diabetes, not just those who have chosen to buy it for themselves since it become available. These users have created word-of-mouth recommendations, including how it has changed their HbA1c for the better. Certainly many wondered if it was just easy to use and discrete, but not able to significantly improving control, therefore of no greater benefit than blood tests. However, clinical tests have shown that using the FreeStyle Libre can help bring users’ HbA1c results into range.
‘Time in range’ is a phrase gaining more credence as a measure of good control, it being an indicator of better control. And credit to them for creating a product that’s actually fun to use. A quite swipe – like scanning a tin of baked beans in the supermarket – and you get a reading that shows recent history and your trend, going up or down or – with any luck – stable. Another boon has been parents being able to go into their sleeping child’s room at night and scan them without waking them or prick their fingers to do a reading.
CGM was seen as being extremely elite, with nothing in the middle between BGM (blood glucose monitoring) and CGM (continuous glucose monitoring). Abbott wanted to take the best bits of CGM to create a more gentle technology, a little less assertive than CGM (where the alarms can get a little wearing), and not as hard for clinicians to use either in terms of data download and analysis, as the data from some CGMs being hard to interpret at first. A simple line tracking glucose results on the Libre’s reader is easy to understand and can lead to improved engagement by the user with their diabetes control, leading to improved outcomes in the form of reduced HbA1c. And it has to be said that Abbott’s middle road has had considerable uptake due to being rather fun and easy to use.
Extended range
Looking back it’s as if the initial purveyors of CGM – Medtronic and Dexcom – had warmed up the market place for Abbott, but now they can now bask in the reflected glow. Both Medtronic with its Enlite sensor and Dexcom’s G5 are now going out beyond their core base – pump users – to people on multiple daily injections (MDI). The point is improved control. If this kit helps you do it, then great. Blood test strips are still fiddly to use, CGM is hard to get hold of and relatively expensive. The FreeStyle Libre has let people dip their toes into the waters of continuous information by getting ongoing data, and they liked it. Most felt that the cost investment was worth it.
Abbott’s stated aim from the launch of the FreeStyle Libre system has been to eliminate the need for routine finger prick testing, although many people feel that performing a blood test confirms that their technology is accurate. Harris counters, “We’ve assessed 50,000 users who are doing an average of 15 scans per day, i.e. three times more than the minimum recommended by European guidelines. They spend less time is in both hypo and hyper states and therefore improve their glucose control overall.”
In other words, accuracy is there without calibration. But isn’t the FreeStyle Libre system more costly than blood testing? Why would the NHS fund it? In fact you get much more data from a sensor. A reading is reported every 1 minute and records every 15 minutes, so long as you swipe at least every 8 hours — if you don’t, you might as well stick to blood tests – it’s the on-going insights that really drive better control. Adds Harris, “Better control also means potentially fewer hospital admissions, which are costly, as well as an overall cost saving to the NHS where 80% of diabetes costs are due to treating diabetes complications. Any reduction in those is meaningful.”
As for availability, Harris reconfirms, “The FreeStyle Libre system will be available in all four countries in the UK and we are working closely with local health economies to get it on formularies. The focus is people with diabetes who are intensively-using insulin. However, it may take some time to get listed and make its way onto local health economy formularies.”
The FreeStyle Libre system is made in the UK, so it’s a great export story too, with more jobs coming soon to help meet the demand. Globally there are more than 300,000 users in 35 countries with the FreeStyle Libre system currently reimbursed in 17 of these.
Diabetes UK has welcomed the announcement that flash glucose monitoring technology will be made available on prescription across the UK, calling the decision ‘life-changing’. Chris Askew, Chief Executive of Diabetes UK, says: “This announcement is fantastic news: Not since the transition from urine testing to finger-prick testing has there been such potential to transform the lives of people living with Type 1 and Type 2 diabetes through technology. Flash glucose monitoring can free people living with diabetes from the pain and rigour of frequent finger-prick testing, and puts them in greater control of their condition. In doing so, it has the potential to help prevent a host of devastating long-term complications. Today’s decision is testament to the commitment of campaigners, clinicians and policy makers to making this technology available. The challenge now will be that everyone who could benefit from this technology is able to access it where they live; Diabetes UK will be looking to local decision makers to ensure people living with diabetes get proper access to this potentially life-changing technology.”
*According to Abbott, Flash Glucose Monitoring is a new category that is designed as an affordable and easy way to generate the dense glucose data needed for an insightful glycaemic picture. What makes flash glucose monitoring unique is the quick scan of the reader over the sensor to collect glucose data. Another key feature of the flash glucose monitoring system is the small and fully disposable sensor that lasts up to 14 days and that automatically measures, captures and stores glucose data.
Implantable CGM
Roche’s Accu-Chek brand is soon to bring Senseonic’s Eversense CGM to the UK and other countries in Europe, the product having gained CE clearance earlier this month (September 2017).
A recent survey of 17,317 Type 1 diabetes (T1D) individuals in the American-based T1D Exchange registry reported 41% of the 1,662 respondents who were using CGM at registry enrolment discontinued use during the first year. Reasons for discontinuation include skin reactions, alarm fatigue, discomfort, size of devices and technology issues. Current commercially available CGM systems last between 6 to 14 days and are worn externally. The new implantable Eversense CGM system may provide relief from many of these problems and therefore has the potential to improve compliance for users.
The Eversense system continually measures interstitial fluid glucose levels in adults with diabetes for the operating life of the sensor, which is indicated at 180 days (six months). Although the CGM is implanted the uses wears a reader on the skin above the technology, including glucose trend information and includes alarms for the detection and prediction of episodes of hypo and hyper. This data can be transmitted via an app to a smartphone. The aim is that the system will allow users to manage their diabetes with more flexibility, confidence and discretion. The system needs calibrating twice a day with a blood glucose reading.
The Eversense sensor is inserted in a clinical environment where a 5‒8 mm insertion is made in the upper arm under a local anaesthetic). A small pocket is then created 3‒5 mm under the skin into which the sensor is inserted using a special insertion tool. The insertion is then closed with steri-strips.
The implanted sensor is powered wirelessly by the transmitter placed on the arm directly over the sensor. The Eversense mobile app can send the data wirelessly to a cloud-based application allowing the data to be shared with the caregiver or diabetes healthcare team. The sensor is removed in the clinic using local anaesthetic. Another sensor can then be inserted into the same place. Alternatively, the incision is closed using steri-strips if a new sensor is to be inserted at a fresh location.
Once implanted, the implantable Eversense sensor provides accurate glucose data for up to 180 days. The transmitter placed on the skin above the sensor is removable and rechargeable. It sends data to a smartphone app. The Eversense smart transmitter is low profile, lightweight and easy to wear under clothing, being secured to the skin using adhesive tape. It is water resistant too. The smart transmitter powers the sensor, calculates the glucose values and direction and rate of change. It also provides on-body vibration alerts even the smartphone is not nearby or switched off. The Eversense mobile app displays the sensor glucose data from the smart transmitter, displaying the current glucose value every 5 minutes and displays where the glucose is headed and how fast.
Eversense implantable CGM will become available in due course, and we will we report further when it is available.
Would you wear CGM?
Diabetes devices (insulin pumps, continuous glucose monitors are associated with benefits for glycaemic control, yet uptake of these devices continues to be low. Some barriers to device uptake could be addressed, but little is known about which barriers and which patients to target.
A survey of 1,503 adult T1D Exchange participants unearthed attitudes (general and diabetes-specific), and barriers to device use and reasons for discontinuing devices. The most common barriers were related to the hassle of wearing devices (47%) and disliking devices on one’s body (35%). CGM users (37%) were older than nonusers and had had diabetes for longer as well as having more positive attitudes to technology than nonusers, however the youngest age-group in the study (18–25 years) had the lowest CGM use (26% vs. 40–48%) and insulin pump (64% vs. 69–77%) uptake. They also reported the highest rates of diabetes distress and highest HbA1c levels.
The study concluded that efforts to increase device use need to target physical barriers to wearing them. Because young adults had the lowest device uptake rates, highest distress, and highest HbA1c compared with older age-groups, they should be the focus of future interventions to increase device use.
Diabetes Device Use in Adults With Type 1 Diabetes: Barriers to Uptake and Potential Intervention Targets, a study by M. L. Tanenbaum et al. Diabetes Care 2016 Nov; dc161536. https://doi.org/10.2337/dc16-1536